- Регистрация
- 17 Февраль 2018
- Сообщения
- 25 397
- Лучшие ответы
- 0
- Баллы
- 2 093
Offline
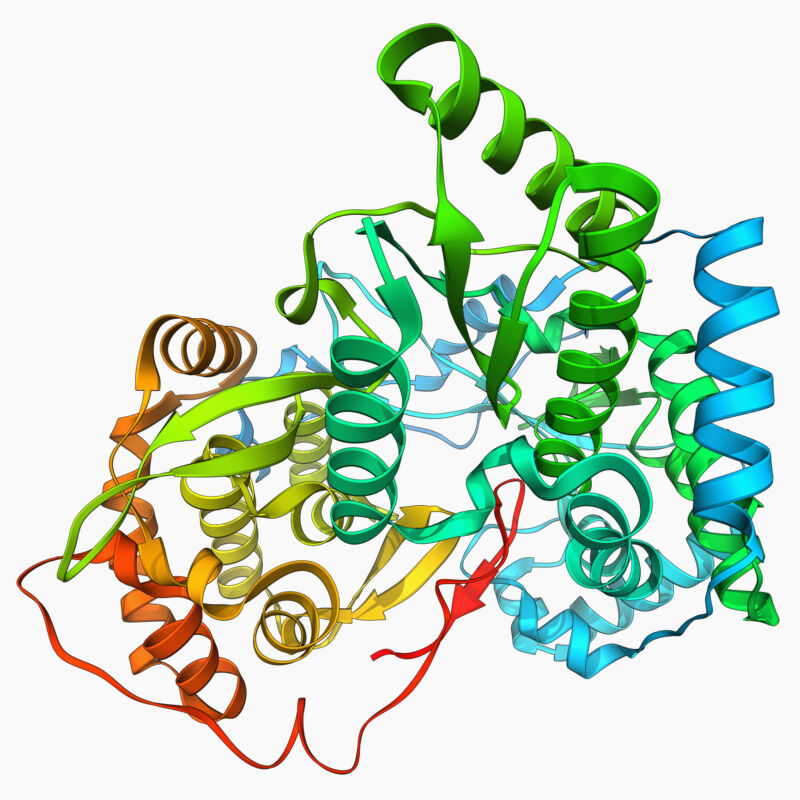
Enlarge (credit: LAGUNA DESIGN)
For most proteins, structure is function. The complex three-dimensional shapes that proteins adopt create folds and pockets that can accomplish the remarkably improbable: driving chemical reactions that would otherwise never happen or binding to a single chemical inside the complex environment of a cell. Protein structure is so important that there's an entire discipline, along with several well-developed approaches, to figuring out what a protein looks like when it's all folded up into its active state.
But that's only most proteins. Scientists have also found a growing catalog of intrinsically disordered proteins. Rather than having a set structure, intrinsically disordered proteins seem to have entire sections that can flap around in the breeze of Brownian motion and, yet, were critical to the protein's structure. People haven't been sure whether these proteins temporarily adopted a specific structure to work or the disorder was critical for function.
Now, a new paper describes a case where two intrinsically disordered proteins induce specific structures in each other when they interact. And Google's new AlphaFold AI software was critical to figuring out that structure.
Read 14 remaining paragraphs | Comments
